Cristal Therapeutics announces a publication in ‘Chemical Science’ on CliCr® technology platform, comprising a new class of superior copper free click reagents for conjugation of small molecules, biologics, nanoparticles and other moieties
- Flagship chemistry journal describes the development of TMTHSI (CliCr®) as a superior click reagent
- Cristal applies the CliCr® platform to the rational design of CriPec® nanomedicines, also enabling modular CriVac® vaccine product development
- CliCr® can be partnered or licensed as a stand-alone technology
- Cristal is actively seeking partners for CliCr® for a variety of applications
Maastricht, The Netherlands, 29 September 2020 – Cristal Therapeutics, a Dutch phase 2 clinical-stage pharmaceutical company developing targeted nanomedicines for the treatment of cancer and other diseases with high unmet patient need, announces publication in Chemical Science, the flagship journal of the Royal Society of Chemistry, of an article on the development of TMTHSI as part of the CliCr® platform providing fast and versatile conjugation tools.
For the optimal performance of CriPec® nanomedicines, it is essential to be able to attach a broad range of small molecule active agents and large molecular entities, biologics, to CriPec® nanoparticles.
The published research1 reports the development of a convenient and versatile fast-reacting molecular entity for gluing very different compounds in a strain-promoted azide-alkyne cycloaddition click reaction to the nanoparticles, as well as a collection of linkers to attach the widely varying active small molecules and biologics. Next to the already demonstrated examples, many additional applications are foreseen such as the construction of antibody drug conjugates in aqueous environments with faster kinetics that is essential for these delicate constructs.
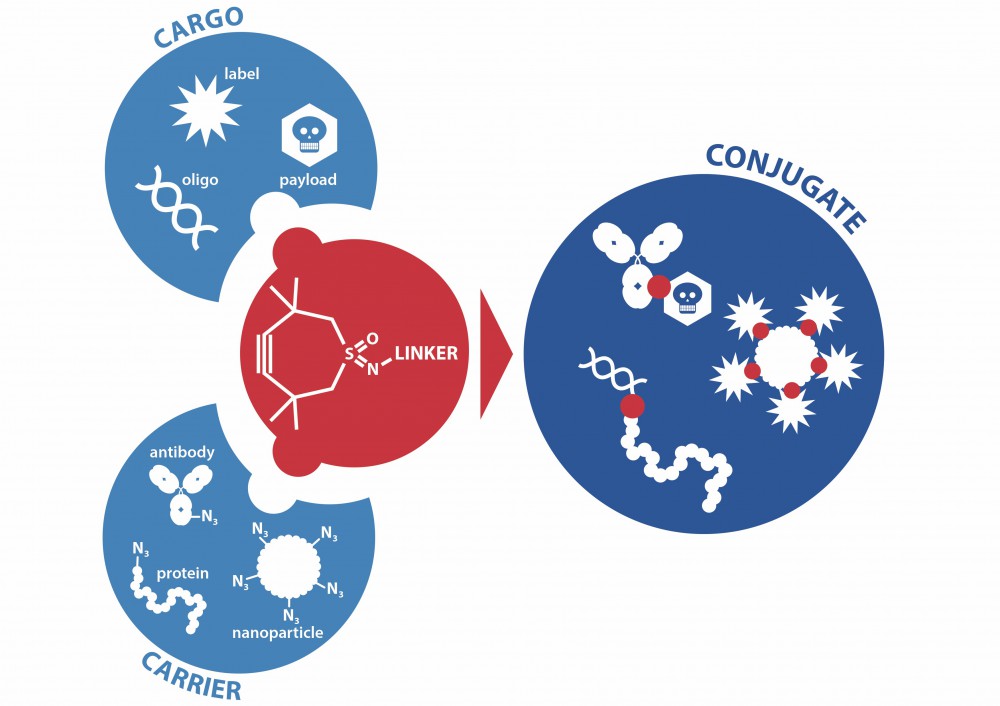
CliCr® is also used to generate virus mimicking nanoparticles. CriVac® is a unique antigen carrier platform based on CriPec® nanoparticles that, in contrast to viral vectors, do not convey a bystander immune response. CriPec particles’ size resemble a virus and the desired numbers of antigen displayed on its surface are controlled via CliCr®. CriVac® mimics features of a live virus in a tailored manner to induce immunity safely, efficiently and solely to the displayed antigen, offering a prophylactic vaccination strategy which will be readily adaptable to different pathogenic treats.
The very attractive functionalisation possibilities, combined with its versatility, great reactivity and small size offer multiple opportunities for CliCr® reagents to become the new standard for non-copper catalyzed click reactions in a multitude of applications.
Dr Cristianne Rijcken, CSO of Cristal Therapeutics, stated:
“This new versatile click reagent originates from an intense collaboration between industry and academic partners. For our nanomedicine applications, a fast, cleanly reacting and small click reagent is absolutely indispensable. These demands required the development of a new reagent, which will be highly attractive both for our proprietary applications and for the wider world of the biological, medical and material science applications. This is ground-breaking technology!”
In case you are interested to learn about the CliCr® platform, please reach out to www.clicr.eu or talk to us at the following virtual conferences
- BioPharm America 21-24 September 2020
- Oncology Virtual Partnering 22-24 September 2020
- Bio Europe 26-29 October 2020
- BioTech Showcase 11-13 January 2021
Reference
1. J. Weterings et al. TMTHSI, a superior 7-membered ring alkyne containing reagent for strain-promoted azide–alkyne cycloaddition reactions, Chemical Science (2020)
https://pubs.rsc.org/en/content/articlehtml/2020/sc/d0sc03477k
– ENDS –
About Cristal Therapeutics
Cristal Therapeutics is a phase 2 clinical-stage pharmaceutical company developing targeted nanomedicines for the treatment of cancer and other diseases with high unmet patient need and considerable commercial potential. The Company’s product candidates are based on its proprietary CriPec® polymeric nanoparticle technology platform, which enables the design of customized nanomedicines with superior therapeutic profiles. CriPec®-based products have the potential to provide enhanced efficacy and reduced side effect profiles, thus offering improved disease treatment.
Find out more: www.cristaltherapeutics.com
For more information, please contact:
Cristal Therapeutics
Jeroen van Egmond
Consultant Business Development
T: +31 6 272 048 89
